Lichens, inconspicuous masterpieces
You probably think of the city as a fairly inhospitable place. Sure, there is some life in the gardens and
parks, but obviously not on the barren roads or on walls and roofs of buildings, right? Exposed to the
wrath of the elements, to rain, blistering cold, or to scorching by an unrelenting sun. No apparent source
of food in sight. How can anything eke out an existence in such a place? Well, being a lichen is a great
starting point. Lichens are a group of commonly overlooked, tough, little organisms that thrive in the
most inhospitable of places. On bare walls, on the pavement, on trees, in cold climates, and in hot
climates. They are the unsung superheroes of survival. A dream team, a symbiosis made up of an alga or a
cyanobacterium and a fungus, called the photobiont and mycobiont, respectively. The alga or
cyanobacterium generates nutrients by photosynthesis, and the fungus supplies essential minerals and
protection from the elements. A seemingly perfect combination, but how did it come to be? Unlike most
relationships, this one did not start with a bit of chemistry and a slight overconsumption of alcohol. In
fact, it did not have one defined starting point, but evolved many times independently. It is a highly
successful way of life, partly because the fungal partner, the mycobiont, produces a whole range of
interesting substances. Not, unfortunately for the more enterprising among our readers, narcotic
substances. Among these substances are, however, compounds with anti-cancer effects that could benefit
us greatly. Lichens, in short, are extraordinary organisms. But how do you find out more about what
makes them tick? And what difficulties do you run into while studying them in the lab? This article will
tell you all about the evolution of, anti-cancer compounds produced by, and ways to conduct research on
lichens, supreme survivalists.

Figure 1:
A collection of lichens growing on stone in Utrecht. Photo courtesy of the authors.
And then there was lichen
Lichens are the textbook example of symbiosis. They are, in that context, omnipresent in biology study
books. But what is a symbiosis? Simply put, a symbiosis is an intimate interaction between members of
two different species. When this interaction has a clear benefit to both, we call it mutualism, as is mostly
the case with lichens. There is evidence in ancient marine deposits that lichen symbiosis first occurred as
many as 600 million years ago. This interaction may have begun as a facultative relationship with marine
algae and cyanobacteria in shallow marine ecosystems, at a time before the colonisation of land by
vascular plants. Within this ecosystem the photobionts and mycobionts-to-be must have lived in large
numbers and close association with each other, as this is a necessary condition for the evolution of their
symbiosis. The symbiosis most likely started off as a parasitic or commensal
off primary metabolites such as sugars extracted from the algae(1).
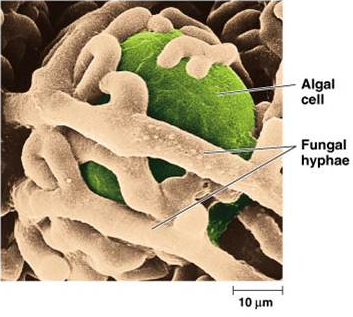
Figure 2:
An algal cell, the photobiont, enclosed by the fungus, the mycobiont. Source (2)
Shady dealings in the light of mutualism
The name of a species of lichen refers to the species of fungus that is part of it. Lichens are in fact
symbionts consisting of both a photobiont, like an alga or a cyanobacterium (in respectively chlorolichen
and cyanolichen), and a fungus, referred to as the mycobiont. They envelop one another closely,
exchanging nutrients (see figure 2). The two types of organisms have developed a highly successful
lifestyle, so much so that one in five of the known fungal species engage in this relationship(3). Genes
coding for the subunits of ribosomal RNA are highly conserved and are often used in studies to
determine ancestry(4).
This means that researchers can compare the sequences of these genes in different
species and determine their relationship. Using sequence data of these genes, obtained from lichenised
and non-lichenised states of currently living species within the fungal phylum ascomycota, phylogenetic
researchers have managed to deduce that many non-lichenised species or orders within the fungal phylum
ascomycota descended from lichenised ancestors(5). Even though 98% of lichen forming species of fungi
belong to the phylum ascomycota, lichens cannot be seen as a monophyletic group(3). This is because
not all lichens are part of the ascomycota, but also because lichenisation has arisen on separate occasions
within the phylum and has also disappeared from some lineages(5). The term ‘lichen' is therefore a
phylogenetically obsolete term. It is, however, a good example of convergent evolution(3). The benefits
for both the photobiont and the mycobiont are clear enough. The photobionts fixate carbon and supply
part of it to the fungus, in the form of simple carbohydrates. The fungus, in turn, facilitates photosynthesis
in the photobiont by positioning it towards incoming light, supplying it with water and nutrition in the
form of minerals and by facilitating gas exchange(6).
Why, then, have many species of fungus abandoned this seemingly perfect symbiosis? The answer to this
question might lie with the lichenicolous fungi (fungi dwelling on or in lichens as parasites or
commensals). Based on morphological evidence, these fungi seem to be related to lichenised species(3,5).
They have shed their lichenised habit but still acquire their carbohydrates from the photobionts of another
lichen. In effect, they steal the reward of the mutualistic relationship from a lichen while not having to
pay the cost of forming the structures necessary to facilitate the photobiont. This parasitism might be the
first step towards no longer feeding from a photobiont at all, and switching to an entirely different
substrate. This serves as an example that mutualistic symbiosis is not always the end of the line but that
the interaction can break down again over evolutionary time(3,5,7).

Figure 3: Lichenicolous fungus (red) growing on a lichen (white/brown). Source (8)
Perks of lichenisation
What sets apart many lichens and their descendants from other fungi, is their wealth of so-called
secondary metabolites. Secondary metabolites sound daunting, but they are simply all substances that are
not purely used to obtain energy or for essential processes such as maintenance of the cell wall or
replication(9). But why do lichens produce so many secondary metabolites? The answer to this question
is closely tied to the special relationship between photobiont and mycobiont. The photobiont supplies
energy-rich carbohydrates to the mycobiont. This ample supply took the strong evolutionary pressure off
the pathways the fungus used to produce carbohydrates on its own. The mycobionts could thus freely
evolve to produce secondary metabolites like antibiotics, and so they did. A famous example of this is
penicillin, which is produced by a member of the lichen-derived fungal taxon Penicillium(5.
Desiccation tolerance
Lichens are also highly resistant to desiccation or drying out, which is a useful trait for an organism
growing on a substrate that retains little water. Unlike plants, lichens do not have a water transport system
and therefore cannot regulate their own water status. They may contain as little as 10-20% of their dry
weight in water when desiccated, to as much as 2000% in some cyanolichens. Most lichens can survive in
their dried up state for months if their environment is stable enough. When the desiccation proceeds
slowly (over several hours) then most lichens can even survive drying up to a water status of 5% or less.
If we lost 95% of our water, we would not fare well, in fact, it would be our farewell. Considering the
stress such circumstances put on any living cell, surviving it is truly amazing. Membranes tend to rupture,
pH becomes more extreme, production of reactive oxygen species (ROS), chemicals that have an oxygen
atom that violently attacks other molecules, increases, and proteins denature. Lichens deal with these
stresses by effectively shutting down their metabolism, keeping their cells turgid with sugars instead of
ions and by synthesising a wide range of antioxidant proteins to neutralise the ROS.
Of course, desiccation resistance is variable among lichens. Species growing in a tropical canopy are
generally less resistant than those growing on exposed tree barks, rocks, or buildings for that matter(10).
Just as important as surviving desiccation is coping with rehydration. If cellular metabolism could not be
restarted after rehydration, the lichen would slowly wither and die. The key aspect in coping with
rehydration is generating the energy needed to get the cell back in working order. Shortly after
rehydration, however, photosynthesis is still shut down, so no energy-rich carbohydrates can be generated
that way. Luckily, the lichen has a back-up plan, a special enzyme that is used to generate energy when
photosynthesis is not yet available. This enzyme's resistance to desiccation is a measure of drought
resistance in lichens(10).

Figure 4: Lichen with apothecia (elevated yellow discs) on a tree. Photo courtesy of the authors.
Lichen reproduction
Lichens are hard to kill, but how do they start their lives? Lichens can reproduce both sexually and
asexually. Most lichens form sexual structures that contain fungal spores, called apothecia. The elevated
yellow discs in figure 5 are an example of these structures. A new lichen can only be formed via sexual
reproduction when a mycobiont spore germinates in the right microhabitat where a suitable photobiont is
available(11). Reproducing in this way is a relatively uncertain investment for the lichen. Most spores
will never grow into lichens, but they can disperse over a long range. This is useful for when the going
gets tough in the lichen's direct environment(12). Asexual reproductive structures of many different
shapes and sizes are also common. Asexual reproduction is a second, safer, investment because the
mycobiont is dispersed with its photobiont in tow which skips the need for relichenisation. A piece of the
lichen simply breaks off, complete with photobiont. These asexual spores or propagules don't disperse as
far as the sexual ones, simply because they are heavier(11). This strategy works best for reproduction
while the going is good in the lichen's direct environment(12).
Why do lichens fare well in the city?
rLichens are often the first multicellular, eukaryotic organisms to venture into different kinds of barren
uninhabited territory. There are three types of lichens, the epiphytic lichens that grow on plants, typically
bark or branches of trees, the terricolous lichens that grow on the ground, on places where vascular plants
have not (yet) taken root, and last but not least, epilithic lichens that grow on stone surfaces, such as the
rocks that are left by a retreating glacier or the walls of a building(13). It is this final category that is most
typical for the city. What makes them thrive in such a barren and harsh environment? And why are they
ecologically significant in general?

Figure 5: Examples of both epiphytic (left) and epilithic (right) lichen, respectively. Photo courtesy of the
authors.
Living off a rock
It is important to appreciate that cyanolichens not only get carbon from the photobiont, but also nitrogen,
because their symbiotic cyanobacteria have the capability to fixate nitrogen. Even those lichens that
cannot fixate their own nitrogen can often do well in the city because of another urban phenomenon and
nitrogen source: bird - mostly pigeon- droppings(13). Nothing can live on just water, carbon and nitrogen.
Different kinds of minerals are also essential to life, such as calcium, magnesium and potassium. These
are supplied by the mycobiont, which partly extracts them from the rock the lichen lives on. It does so by
adhesion to the rock and excretion of small organic acids such as oxalic, citric and lactic acid, that slowly
dissolve the rock(13). The minerals are then taken up in solution. This is a very slow process, but lichens
can absorb most of the minerals they need from rain and settling dust. Given enough time, however,
lichens and other microorganisms that also scrounge up minerals from rock can bring down stone
buildings(13,14).
Leading life to new lands
The combined ability of lichens to mobilise minerals out of rock, fix nitrogen and photosynthesise, makes
them capable of pretty much living on air, stone, dust and a little light. As indicated before, they're also
highly resistant to desiccation. All this often makes them the first multicellular eukaryotes to colonise
newfound territory. There is even fossil evidence that lichens were the first terrestrial eukaryotic
organisms(15). This is not unlikely, since the living conditions on the barren land around 500-600 million
years ago must have been harsh and lichens are tough as nails(15,16). And possibly as snails, though not
quite as slimy.
By fixing nitrogen and minerals and loosening up hard substrates, lichens have paved the way for plants
to colonise the newfound land, and they are still doing so. There is even phylogenetic evidence that
lichen-like symbiotic relationships were the starting point for interactions between fungi and plants, a
relation that is actually quite similar to the one within lichens. Plants grow much faster than lichens in the
suitable environment their predecessors created, and literally overshadow them(14-17). However, walls of
buildings and the humans that live in them generally don't allow for much further succession. Therefore,
lichens thrive, relatively undisturbed except for the occasional slaughter enacted by high-pressure
cleaning or scrubbing.
Secondary metabolites
The topic of evolution neatly covered the fact that many successful fungi are descendants of the
mycobiont in a lichen symbiosis. The mycobiont, free from the stress of basic metabolism (the photobiont
busies itself with the hurly-burly of basic energy needs), had a lot of freedom to produce compounds with
interesting functions. These compounds can fight bacteria, protect against radiation or desiccation and
much more(9). By (almost) pure coincidence, many of these compounds are also useful for us. It turns
out that lichens produce many compounds that have anti-cancer properties. Let us see what interesting
possibilities lichen offer us in our battle against disease.
Usnic acid: major anti-cancer compound
The discovery of the anti-cancer properties of usnic acid in 1975 was one of the first major boosts to the
research effort into the use of lichen products against cancer. When mice with lung tumors were treated
with it, they lived 32-52% longer than the control group(18).
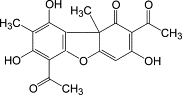
Figure 6: Usnic acid, one of the compounds with anti-cancer properties produced by lichens. Image taken
from source (19).
How exactly the compound works is unknown, but it seems that it somehow hinders cell division. Not all
cancers are alike, however, so the question remains if usnic acid is truly effective against a wide variety of
cancers. The answer to this question is a tentative yes, as a recent study confirmed that usnic acid is
effective in inducing apoptosis and inhibiting cell proliferation in nine different types of cancer cells. A
large effort is still required to translate this compound into a medicine, but the effectivity is there(20).

Figure 7: The lichen Psoroma hypnorum, a member of the genus from which the prostate
pannarin was extracted. The brown circles are conidia. Adapted from source (21).
Pannarin prostrates prostate cancer
An even more interesting compound due to its specificity is pannarin. In 2006, researchers extracted this
compound from a lichen of the genus Psoroma and tested its effectiveness against prostate cancer. As
many men suffer from prostate cancer at one point in their life, compounds that specifically combat it are
valuable(22). Pannarin had no effect on healthy prostate epithelial cells nor on lymphocytes or
hepatocytes in the concentrations tested, but a noticeable effect against malignant prostate cells was
observed. At certain concentrations, only 20% of the prostate cancer cells survived, while virtually all
healthy prostate epithelial cells survived. The effect at this concentration was seemingly caused by an
increase in apoptosis, since caspase-3, an important protein in controlled cell death, was much more
active in these cells. Pannarin is therefore an interesting candidate for further development as an anti-
cancer drug(23).
The last compound we would like to draw your attention to is rather well-endowed in the naming
department. It goes by the name of 16-O-acetyl-leucotylic acid. Its function can be summarised in short as
a potent fighter of leukemia. In tests to determine its effects on the proliferation (i.e. cell division) of
leukemia cells, a significant reduction was found. A promising avenue for further development(24).
What this all shows us is that lichens produce a wide range of compounds that could help in the fight
against cancer. Usnic acid, pannarin and 16-O-acetyl-leucotylic acid all exhibit antitumor effects and
there are many more substances to be functionally characterised(9). The question that remains is how
experts investigate lichens to come to these conclusions. The following is a lichening fast primer in lichen
research practices.
Research
We have established that lichens are hopeful candidates for cancer medication. The question, then, is how
research is performed on lichens to unravel their secrets. Their nature immediately reveals some major
hurdles in lichen research. Slow growth is a problem, because researchers want results and they want
them quickly(25). In addition to slow growth, the fact that there are two separate organisms is a hurdle. If
you want to study what genes are turned on or off when the mycobiont and photobiont come into contact
with one another, you have to culture the organisms separately and then persuade them to become a lichen
once again. In short, reinstituting the lichen growth form is a problem that needs to be overcome. Two
major questions researchers faced were thus how to increase the speed of their experiments and how to
motivate the separate bionts to restart their loving relationship. Well then, how do they do it?
Growing bionts in the lab
In order to grow the lichen mycobiont or photobiont in the lab, you invariably need to collect a lichen
specimen. After collecting, it is washed with water and homogenised with a mortar and pestle. The
mixture is then passed through two sterilised filters. It is deposited in a small vial with sterilised water and
centrifuged, which deposits the lichen cells at the bottom and kills bacteria. These cells are then
transferred to some medium (water with nutrients) and plated onto solid medium (water with some
nutrients and a jelly-like substance called agar) in a petri dish. This then needs to be grown for five
months in continuous light. After five months you obtain a plate with parts that are all mycobiont and
parts that are all photobiont. You can then prick any of the two with a sterile toothpick and transfer this to
a new plate. You have now isolated only the mycobiont or photobiont. Figure 9 shows an isolated,
cultured mycobiont and the lichen form as found in the wild. You have inserted very few cells on the new
plate, so they have to grow for two months until you can use this plate. Now you can take the cells on this
plate, and perform all sorts of experiments on them: grow them on different media, to see how fast they
grow on those media, or combine the separate bionts to reinstitute the lichen form. The growth phase,
however, is five months. In total, this has already taken twelve months, hardly a competitive time frame
in research, especially when you want to look at the production of specific compounds(26). What can be
done to speed up this research?

Figure 8: The left photo shows the cultured mycobiont of the lichen Xanthoria elegans, the right photo shows the lichen as
found in the wild. Adapted from source (27).
The need for lichen speed
One solution to the problem of growth speed is the cloning of a gene from a lichen into another, fast-
growing, fungus. Recall that many current fungal lineages were once lichenised, so they are very close in
terms of genes and regulatory systems. This is a great advantage, since a gene from a lichen can be
expressed in another fungus under the control of its own promoter, and interesting compounds from the
lichen can thus be produced much more quickly. In research, tried-and-tested systems are often preferred.
One such system is the pTAex3 system. In this plasmid system, the gene you want to express is put under
the control of an alfa-amylase promoter. This promoter is normally responsible for expression of genes used
in digestion of starch, and is activated in the presence of starch. The system is used in a special strain of
Aspergillus oryzae, the fungus used in fermenting soy sauce. This strain has a deletion in the argB gene,
which is responsible for synthesis of the amino acid arginine. Without this essential amino acid, the
fungus cannot live, so it needs to be provided in the medium. In the pTAex3 plasmid, the working argB
gene is included. When you combine an Aspergillus oryzae mutant (without argB) with the correct argB
gene in the pTAex3 system, this organism will be able to grow in medium without arginine. What this all
means is that the pTAex3 system allows you to control when the lichen gene is expressed (by growing on
starch or another nutrient) and to test whether the lichen gene entered the cell at all (by the ability or
inability to grow without the presence of arginine). The exact method of constructing a DNA vector with
the genes you want expressed, to get products such as anti-cancer compounds, is outside the scope of this
article, but when this vector has been made it still needs to enter the cells. Cells do not, however, go
around picking up random DNA all the time. First, the fungal cells need to lose their cell walls. This is
done by adding special proteins (glucanases and chitinases) that break it down, resulting in what is called
a protoplast. Then, the DNA still needs to magically tunnel through the cell membrane. Since DNA is a
bit short on magic tunneling equipment, researchers help it along by adding calcium ions and PEG
(polyethylene glycol) to the cells. This somehow makes the cell membrane permeable to DNA. The DNA
can now be inserted in the genome of Aspergillus oryzae. If all goes well, you have then created a fast-
growing fungus that can make one of the compounds that normally only lichens can produce(28). This is
one of the ways in which the anti-cancer compounds in lichen could be produced economically. It is,
however, still difficult to do this successfully.
The problem with relationships
The lichen mycobiont and photobiont can be cultured separately. The initial problem of obtaining the
lichen form in the lab (called resynthesis) from the separated mycobiont and photobiont is evident. A
seemingly important landmark was the discovery in 1966 that what a successful relationship really needed
was a bit of a dry spell. Not an absence of intercourse, but a stress on the mycobiont to connect with the
algal or cyanobacterial partner. In too moist an environment, the two organisms can and indeed do live
separately in the lab. They grow on the same plate, but not in the symbiotic form as a lichen. In a dry and
low-nutrient environment, however, the mycobiont and photobiont reinstituted their symbiosis(29). Later
studies have noted that it is primarily the nutrient-poor conditions that play a crucial role in
reestablishment. Now, the mycobiont and photobiont are grown on discs of gauze with pores in them.
Pores, which are large enough for nutrients from the medium to pass through them, but small enough for
the fungus to be unable to grow into the medium. This also makes it easy to transfer the colonies to
different plates. See figure 11. The mycobiont is starved for some time, e.g. two weeks, and then the
photobiont is added. This allows the lichen form to be created in the lab(25,30).
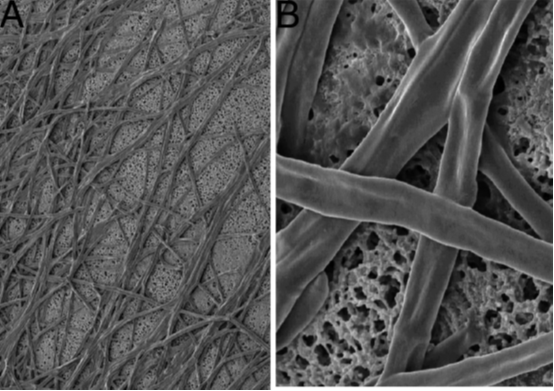
Figure 9: The left photo shows fungal hyphae growing on a cellulose-acetate disk (i.e. a circular gauze). The right photo is
further zoomed in, and demonstrates that the mycobiont does not grow into the pores. Adapted from source (25).
Researchers have dealt with the problems of reinstituting the lichen form in the lab by exposing the
separate bionts to low nutrient conditions, and by growing them on separate circles of gauze so they can
be easily transferred. The growth of lichens in the lab is still very time-consuming, but the inclusion of
lichen genes in fast-growing fungus offers a hopeful perspective for the future, especially for the
production of useful lichen substances such as pannarin or usnic acid.
Conclusion
Lichens are extremely versatile and truly awe-inspiring. That is the simple message of what you have
read. They have evolved independently numerous times and might have been the forerunners to the plant-
microbe interactions that are omnipresent today. They can survive extreme desiccation and rehydration.
Reproduction is often accomplished by a piece of them simply breaking off and being transported to
greener pastures, or, in the case of lichens, more barren rocks. Because the mycobionts were free from the
burden of producing primary metabolites, they have produced a wealth of secondary metabolites with a
wide range of functions. Coincidentally, some of these substances are very potent anti-cancer compounds.
By introducing the genes for these substances into fast-growing fungi, a wealth of new candidates for
cancer treatment will become available. Truly, these are naturef's unlichely superheroes.