Bioremediation against nuclear radiation
The solution of the future
Our cities keep on becoming larger and larger, resulting in an increasing energy demand.
One of the current solutions for this situation is the building of nuclear plants, which
provide us with energy. Besides all the benefits, these plants entail major disadvantages
as well. The disasters at Chernobyl and Fukushima show that ionising radiation can
cause major damage to organisms and the environment. Ionising radiation is a form of
radiation which is capable of exciting or ionising atoms by transferring energy. Our
environment is not only being polluted with radioactive waste during nuclear disaster,
but on a smaller, continuous scale as well. Radioactive waste is constantly being formed
in power plants, research, medical care and other industries. Preventing this pollution is
important, but so is removing it. This is where biology could come to the rescue. One of
the approaches currently studied is bioremediation, the removal of pollution from the
environment with the help of organisms. Nuclear power plants might be the solution for
the increasing energy demand, but bioremediation could be the solution for the potential
collateral damage they cause.
Nuclear disasters
Nuclear disasters can cause widespread pollution and havoc. The best known nuclear disaster
in history took place in 1986, when one of the nuclear reactors of Chernobyl exploded. Large
amounts of radioactive products were released directly into the environment, adversely affecting
many people due to radiation exposure. It is unclear how many people died from the direct
effects of radioactivity exposure, but an immense area became uninhabitable as a consequence
of the contamination with nuclear waste (1; 2). Nuclear waste is similar to other sorts of waste,
except for the fact that it often consists of heavy metals and is a source of ionising radiation.
Unfortunately, the Chernobyl accident was not the only nuclear disaster. Similar nuclear
accidents occurred in Fukushima in 2011, Three Mile Island in 1979 and Tokaimura in 1999(1).
But the problems are not limited to the land. The contamination of the earth's water bodies is
another cause for alarm. Nuclear power plants often generate radioactive wastewater containing
heavy metals(3). Toxic concentrations of these metals accumulate in living organisms, since
heavy metals are not biodegradable. While heavy metals are intrinsically toxic, radioactive
heavy metals are even worse(3,4). Moreover, the half life of radioactive materials like uranium-238
is extraordinarily long; about 4500 million years (5). Although about 95% of all radioactive
waste is formed in nuclear power plants, a small but still significant amount originates from
research, medical care and other industries(6). This means that not only the areas around the
power plants are being polluted, but also areas within our own cities.
Nuclear radiation: what does it entail?
Let us take a look at what radioactivity actually is. Radioactivity can be defined as the
spontaneous decay of a radionuclide, which is a synonym for a radioactive isotope,
accompanied by the emission of ionising radiation. This form of radiation is capable of exciting
or ionising atoms by transferring energy. When energy is indirectly passed on through rays
which are absorbed by the atoms of the matter, it is called electromagnetic radiation. In
particulate radiation, conversely, electrons are dislodged when charged particles directly hit
atoms with kinetic force. Although the methods by which the energy is transferred differ, both
types of radiation cause the atoms to become ionised. This ionisation can break chemical bonds
and cause the formation of free radicals(7).
Harmful effects of radiation
It will not come as a surprise that ionising radiation can have disastrous effects on the human
body and organisms in general. Radiation can cause damage to cells, which can lead to cell
death, or in some cases make the affected cells malignant. This implies that they can increase
the risk of tumor formation. As long as the degree of cell death remains limited, this loss can to
some extent be compensated by the regenerative capacity of the body. When too many cells
are damaged, however, essential processes may become restricted, with all the consequences
that entails. At DNA level, inflicted damage can result in cell division errors if not repaired
properly. The accumulation of such errors can lead to tumor formation. Radiation exposure
causes some other health risks as well. Prenatal exposure can for example lead to brain
damage in an unborn child. In addition, hereditary effects due to radiation overexposure have
been observed in several animal species(8, 9). Figure 1 is an example of the hereditary effects
of radiation on the pale grass blue butterfly. Genetic mutations cause abnormal development of
the wings, resulting in deformed or reduced wings.

Figure 1:
Hereditary effects of ionising radiation on the pale grass blue butterfly, after the
Fukushima nuclear disaster in 2011. The normal wing development is disturbed, resulting in
deformed wings. For example, these butterflies have unequal hindwing size, folded and rumpled.
Source.
Radiation resistance
There is an immense variation in radiation resistance amongst organisms. The lethal dose for
insects, for example, can be up to 100 times higher than that for vertebrates. A possible
explanation for this fact is that a cell which has a relatively high mitotic activity and low
differentiation, is more sensitive to the effects of radiation. Vertebrate cells divide remarkably
more in their adult life phase than the cells of an insect.The variation of different species for
lethal effects of ionising radiation is depicted in figure 2. The figure shows the variation on a
logarithmic scale. Depicted are the LD50 values, which is the dose at which 50 percent of the
animals die. Note the LD50 for dogs, which represents the vertebrates, is about 100 times lower
than the LD50 for Drosophila, which represents the insects(10).
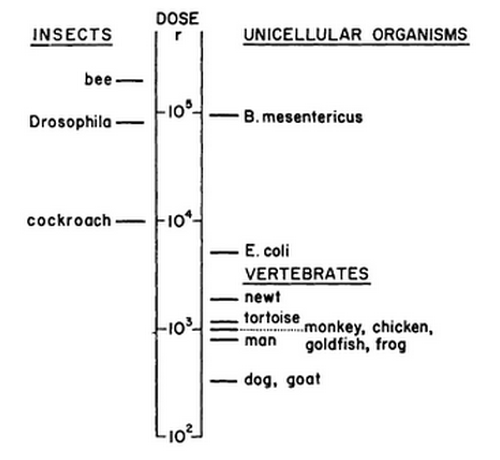
Figure 2:
There is a wide variation in the sensitivity for radiation between different types of organisms. This figure shows the LD50's of different organisms on a log scale.
Source.
The insects' radiation resilience is far from exclusive. Other organisms are also highly resistant.
A totally different type of organism which shares this quality, is the bacterium Deinococcus
radiodurans(11). How these bacteria attain this resistance is quite extraordinary. When
compared with bacteria that are more sensitive to radiation, it is found that they suffer similar
damage. There is no difference in the susceptibility of the DNA to radiation damage. The most
occurring consequences of exposure to ionising radiation are double strand DNA breaks and
damage to the base sequence of the DNA. However, the amount of double strand DNA breaks
in D. radiodurans is similar to other, more sensitive bacteria. How is it, then, that D. radiodurans
is so miraculously radiation resistant?
A likely explanation for D. radiodurans' notably high resistance to ionising radiation is that it has
exceptionally good protection mechanisms. Particularly those that protect the bacteria's
proteins, since protein damage leads to problems with several important cellular processes,
including DNA repair and eventually can cause cell death. Usually, the total of an organism's
proteins, called the proteome, works closely together with the genome in order to guarantee the
maintenance and replication of the cells. The proteome keeps the DNA of the genome repaired
and the genome in turn provides sufficient high protein levels. So while not directly protecting
the DNA of the genome, a good protein protection mechanism can still keep the genome
functional. It is therefore D. radiodurans' good protein protection mechanism that causes its high
capacity for fixing DNA damage and double strand breaks(12).
It is likely that the loss of protein function is normally caused by oxidative damage. D.
radiodurans' tactic works by preventing the formation of reactive oxygen species (ROS) or
neutralising them. Reactive oxygen species are chemically unstable molecules containing
oxygen(12, 13). These highly reactive molecules can have a wide range of damaging effects.
The ROS-induced damage in cells is called oxidative stress. An organism's own metabolism
produces ROS, but it can also be produced by external factors, like radiation. These ROS can
lead to potentially fatal DNA damage in the form of double strand breaks(12). This high
resistance to radiation makes D. radiodurans highly suitable as a component in the process of
cleaning the environment from nuclear waste.
Bioremediation
Managing nuclear contamination in the environment is currently a difficult and costly endeavor.
One option is to keep the contamination within the contaminated site. This basically means
leaving the contamination where it is, making sure it does not spread, and waiting until the
contamination degrades naturally. Since this does not actually remove the pollution, the
contaminated area will be unusable for a long time. Also, barriers need to be constructed,
maintained and monitored to prevent the contamination from spreading. Another option is to
remove as much contaminated material as necessary to make the area suitable to inhabit again.
However, this is often very labour-intensive and expensive. It involves removing topsoil, leaves,
pavements and other contaminated material. Depending on the size of the contaminated site
and the severity of the contamination, this may generate large amounts of waste. The
contaminated material then needs to be transported to suitable storage sites. There are a
couple of methods to separate the radionuclides from the rest of the contaminated material,
such as extreme heating or chemical treatment. The radionuclides can then be stored apart
from the rest of the materials. However, these methods are often costly and/or not efficient(14,
15).
A new and promising approach in the process of removing nuclear waste from the environment
is bioremediation. Bioremediation can be defined as the process in which a contaminated area,
such as a city polluted with radioactive waste, is at least partly purified with the help of
organisms or organic materials. Bacteria, plants and algae are only three examples of
organisms which have the ability to clear pollution. Some of the methods for which these
organisms can be used, are bioprecipation, bioaccumulation and biosorption, respectively.
These bioremediation methods generally have lower costs, generate less waste that has to be
stored and are more efficient than the conventional methods (15, 16, 17).
A range of methods and organisms
Bacteria - bioprecipitation
As mentioned, a large problem is the contamination of the earth's water bodies. In
bioprecipitation, soluble components are converted into solid components with the help of
organisms, facilitating their removal from the water. Certain bacterial cells express an enzyme
called nonspecific periplasmic acid phosphatase (PhoN), which enables the bacteria to
precipitate heavy metals quite effectively. This enzyme first hydrolyses organic phosphates,
after which inorganic phosphates are produced. Subsequently, the inorganic phosphates can
interact with the heavy metals. During this process, the metals become precipitated on the cell
surface, as insoluble metal phosphates. Eventually the bacteria sink to the bottom, and can thus
be easily collected and removed. With the aid of these bacteria, it is thus possible to remove
nuclear waste from aqueous solutions. An additional advantage of this method is that it is
effective even when low concentrations of pollutants are present(17).
The problem with precipitation of nuclear waste is that most bacterial species can barely
withstand radiation. This can be solved by using D. radiodurans, since these bacteria have a
high resistance to radiation. Moreover, D. radiodurans bacteria are not pathogenic, which is an
important feature regarding safety(11).
Although D. radiodurans does not naturally produce
PhoN, the PhoN gene can be introduced in a wild type D. radiodurans. By doing so, a
genetically engineered clone can be produced which is non-pathogenic, radiation resistant and
has the ability to effectively precipitate heavy metals. An essential factor is that the introduction
of the gene does not affect resistance to radioactivity.
An important factor which can determine the efficiency of the precipitation process, is the
amount of enzyme produced by the bacterium. This can be enhanced by using the radiation
inducible promoter Pssb, which is actually already present in a wild-type D. radiodurans. By
inserting Pssb in front of the PhoN gene, acid phosphatase activity increases significantly(11).
Currently, bioprecipitation is mainly used in a batch process. In this process, the recombinant
bacteria are mixed in a solution, then left alone. The precipitate is harvested after a certain
amount of time. However, the precipitation process could be improved by using a flow-through
process instead of a batch process. During a flow-through process, the bacteria are immobilised
in gel inside a column and the solution is pumped through the column. This concept constantly
refreshes the solution the bacteria come in contact with and functions similar to a filter in a
swimming pool. A flow-through system might therefore be more effective and efficient in
precipitating waste than in the batch process where the bacteria and the solution are mixed only
once. Unfortunately, the precipitate tends to clog the column, blocking the flow of the solution
and reducing precipitation efficiency. Until this clogging problem is solved, batch applications
are more efficient than flow-through filters(18).
Plants - bioaccumulation
Although a very promising option, bacteria are not our sole solution in the process of
bioremediation. Another useful option is a form of bioaccumulation called phytoremediation,
which is the use of plants to remove pollutants from the environment. It is called phytoextraction
when a plant absorbs and incorporates the pollutants in its biomass. Phytoextraction can be
used to absorb heavy metals, pesticides, radioactive elements, and more from the environment.
It can be used on both land and in (fresh)water. However, plants differ in what type of pollutants
they can absorb. As mentioned before, high concentrations of heavy metals are toxic to most
organisms and radioactive heavy metals are even more so. Plant species used to remove
radioactive waste must therefore not only be resistant to high concentrations of heavy metals in
the soil or in their tissues, but also to radiation. Toxicity is therefore one of the most important
limiting factors when using living plants(19, 20).
Apart from being resistant to radionuclides, such plants also need to be able to effectively
absorb large quantities of these radionuclides. First of all, the plant needs to have the ability to
absorb the desired pollutant. This partly depends on the plant's natural habitat, the environment
and the soil at the polluted site. A plant that normally grows in marshlands will not survive long
enough to be effective in the desert. Environmental factors can also influence the solubility of
the metals and thereby affect the plant's uptake. The ability to grow fast and quickly assimilate
biomass is also desirable, since this improves the rate in which radionuclides can be
incorporated into their biomass. A high root biomass is desirable as well, since this often means
that there are more and/or longer roots, which improves absorption(19). Once the plant has
accumulated enough radionuclides, it can be harvested. The biomass can then be processed in
multiple ways to reduce the volume, such as composting, compacting or combusting(21, 22).
But how do plants absorb these radionuclides and perhaps more importantly, why? Plants that
actively take up large amounts of heavy metals are called hyperaccumulators. They are better in
the uptake, transport, detoxification and storage of heavy metals than non-hyperaccumulators.
While one might expect the difference between these two groups, the hyperaccumulation, to be
genetic, both types of plants often have the same genes. Instead, it is the difference in gene
expression that is important. In most cases, hyperaccumulators overexpress some genes in
comparison to non-hyperaccumulators. For example, to improve the uptake and transport of
heavy metals, hyperaccumulators overexpress genes coding for heavy metal transport proteins.
After and during the uptake and transport, though, the plant still needs to minimise or even
neutralise the toxic effects of the metals. This is mainly done by storing them somewhere they
cannot do harm to the plant and/or by disabling the harmful properties of the metals. Because
the heavy metals can interfere with important (metabolic) reactions in the cytoplasm,
hyperaccumulators for instance move them to less active compartments of the cell, such as the
vacuole or cell wall. In this way, the metals themselves are still toxic, but their effects are
severely limited. Detoxifying the metals is another way to prevent damage. This detoxification is
often done by binding the metal in a complex with small organic molecules. These metal-binding
molecules are called chelators(23).
But what benefits could hyperaccumulation of inherently toxic substances possibly bring the
plant? Some heavy metals, like copper and zinc, are essential for normal plant growth. Others,
such as uranium or cesium, do not have such an obvious function. One explanation is that
hyperaccumulators use these metals as a form of defence. As mentioned before, high
concentrations of heavy metals are toxic to most organisms, which includes herbivores and
plant pathogens. Hyperaccumulators may be resistant to these high concentrations, but for a
caterpillar or snail these are poisonous and potentially lethal. Some plants synthesise their own
toxin, but this is metabolically very expensive. Absorbing toxic metals is therefore likely to cost
the plant far less energy than producing the toxins by itself. Some plants even use a
combination, where the poisonous effects of the inorganic heavy metals and organically
synthesised toxin enhance each other's effect(23).

Figure 3:
Helianthus annuus, or the sunflower, is one of the best candidates for removing radioactive pollution.
Source.
Helianthus annuus, better known as the sunflower, is one of the best hyperaccumulator
candidates for removing radionuclides from water or soil. This plant species is depicted in figure
3. It is among the most efficient phytoextraction plants in general. More importantly, it can
absorb many of the heavy metals most commonly found in nuclear waste, including uranium,
cesium, strontium and zinc(20, 24). Sunflowers have already been used for phytoextraction
outside laboratories and were proven to be very effective. For example, in a single day, young
sunflowers were able to remove up to 95% of the uranium waste outside an old uranium
refinery. After the Chernobyl nuclear disaster, sunflowers were used to remove radioactive
waste from the water in and around the area(24). In 2011, a large project called "Fukushima
Sunflower Foster Parent Project" was started to clean up the radioactive waste in Fukushima
and improve the soil quality. People were asked to buy sunflower seeds and plant as many as
possible in the contaminated area around Fukushima(25).
Algae - biosorption
The ocean poses another challenge. For instance, large amounts of contaminated wastewater
leaked into the ocean at Fukushima. However, most terrestrial plants cannot grow in very salty
environments and gathering radioactive precipitate from an ocean floor would be quite a chore.
The solution to this challenge could be biosorption. This is the use of biomass to adsorb heavy
metals, meaning that the heavy metals become attached to the surface of the biomass. The
material used to adsorb a substance is called an adsorbent. The difference between biosorption
and bioaccumulation is that biosorption is a passive process, whereas bioaccumulation is the
active uptake of heavy metals(26). Algae, and in particular brown algae, have proven to be
targets with a lot of potential. They are abundantly available, their production and use is
inexpensive and they are very effective at adsorbing heavy metals(4).
A polysaccharide called alginate is responsible for the efficient biosorption capacity of brown
algae. Alginate or alginic acid is a porous and permeable gel in the cell wall of brown algae
which can form complexes with heavy metals. This immobilises the metal and binds it to the
algae, so it can easily be removed(4, 26).
The brown algae species Laminaria japonica seems
to be a good biosorbent for radioactive uranium removal. This species can adsorb pollution far
more efficient than commonly used chemical adsorbents. Environmental factors, such as pH,
can influence the adsorption efficiency, but this efficiency can also be improved by making the
adsorbent particles smaller. The adsorption speed is mainly limited by the amount of surface
area with which the pollution can interact. By making the adsorbent particles smaller, the
amount of area per unit of volume increases. Even though this does not increase the maximum
uptake, it does make the uptake process a lot faster. After preparation, the adsorbent is
distributed evenly in the polluted water, so the waste can be fixated on the algae. The resulting
complex of algae and waste can then easily be removed from the water. The weight and size of
the removed algae-bound waste is an important factor, since the bound waste is still radioactive
and needs to be stored somewhere safe after removal from the environment. A large
percentage of the biomass of algae is organic carbon, which can be burned. Ignition can reduce
the weight and size of the algae-waste complex by up to 75%, as is visible in figure 4, whereas
this reduction is negligible for other chemical adsorbents. What remains after ignition can then
be stored in a safe place(3).

Figure 4: On the left, the size of L. japonica before ignition. On the right, the size after ignition.
Ignition can considerably reduce the weight of a carbon biosorbent, as well as its size.
Source.
The best way to keep our environment safe
Whether radioactive pollution enters the environment through a nuclear disaster, leakage or
simply waste disposal, it needs to be removed as quickly and thoroughly as possible to
minimise the damage done. We have seen that there are multiple ways to deal with radioactive
pollution in the environment. Though bioprecipitation with bacteria is effective even at very low
concentrations of pollution, it requires the release of genetically engineered bacteria in the
environment. Bioaccumulation, on the other hand, uses plants in their naturally occurring form.
However, many hyperaccumulators are limited in the pollutants they can accumulate or the
habitats they can occupy. Since biosorption uses dead or inactivated biomass, there is no need
for genetic engineering and there are no habitational limits. In contrast to the previous methods,
it is a passive process. This could negatively impact its speed, the maximum uptake and its
effectivity at low concentrations when compared to the previous, active processes. Furthermore,
phytoremediation is the only method of the three which can be applied on the land.
We can thus draw the conclusion that all three bioremediation methods have their own
strengths and weaknesses and none of them is better on all fronts. Whether bioprecipitation,
bioaccumulation or biosorption is best, depends on the circumstances. What we do know, is that
these bioremediation methods are environmentally friendly, low-cost and efficient methods to
remove nuclear waste from the environment. Our cities are in serious need of energy and
nuclear power plants can provide it, but using the power of the atom entails considerable risks
to our health and our environment. Even though nuclear waste is a very persistent form of
pollution, it can be cleaned up through bioremediation. Therefore, to keep our cities alight and
our lands and waters clean, bioremediation will undoubtedly be a solution of the future.